How Hard and Soft Water affects Bread Rise; is it better to use soft or hard water to achieve the best loaf qualities?

Aqua
Hardness of water is mostly due to the presence of calcium carbonate and magnesium minerals. Hardness is generally reported as the amount in parts per million (ppm) of calcium carbonate. Closely related to water hardness is pH of the water, its degree of acidity. Soft water can be slightly acid, but not always. Hard water is likely to be alkaline, but not always.
Water's Effect on Dough
Both mineral content (degree of hardness) and acidity of the water you use in baking can greatly affect the finished product. In breads, water represents about 40% of the total dough mass. Therefore, if even minor amounts of minerals are in the water, they exert a measurable effect on the dough’s characteristics and the quality of the bread.
Water with a medium hardness (50-100 ppm calcium carbonate) is considered the best for baking purposes. This is because some of the mineral salts have a strengthening effect on the gluten of the dough.
Hard Water
Very hard water (above 200 ppm calcium carbonate) is detrimental because it retards the fermentation process. They do this by tightening or toughening the gluten structure too much. The minerals present prevent the proteins from absorbing water. Corrective steps include:
- An increase in the yeast level.
- A decrease in the amount of yeast food such as sugars/starch used since these contain minerals.
- The addition of acid such as orange juice or yogurt.
- Or a reduction in the amount of added dough improver since these also contain minerals.
- Diastatic Malt supplementation is also recommended.
High pH in hard water is undesirable because the high content of alkaline salts neutralise the normal acidity developed during yeast fermentation. Alkaline waters raise the pH of the dough above the optimum range for enzyme activity. Enzymes act at their optimum at pH levels between 4 and 5. Since the functions of enzymes in dough are significantly affected by the pH of the medium, it is evident that excessively alkaline waters have a detrimental effect on the direction and quality of the fermentation. Alkaline pH can be adjusted by adding acetic acid, lactic acid or mono calcium phosphate. Acetic acid is a naturally occurring acid found in apples, grapes, and oranges. Lactic acid is found in sour milk such as yogurt and buttermilk. Mono calcium phosphate is is one of the common ingredients in baking powder.
Hard water has other disadvantages in that it can leave scale in water heaters, heat exchanges, hot water pipes, and other processing equipment.
Soft Water
Soft waters (10-50 ppm calcium carbonate) are objectionable because they lack gluten-strengthening minerals and tend to yield soft, sticky dough. Also, the low pH of soft waters has an accelerating effect on fermentation. This will require some reduction in fermentation time. While soft water may yield bread with fairly good volume and very even grain, the texture and colour are likely to be poor.
Corrective steps you can take include:
- An increase in the use of yeast food.
- The addition of dough salt.
Total Dissolved Solids
Total dissolved solids (TDS) refers to the amount of minerals, metals, organic material, and salts that are dissolved in water volume.
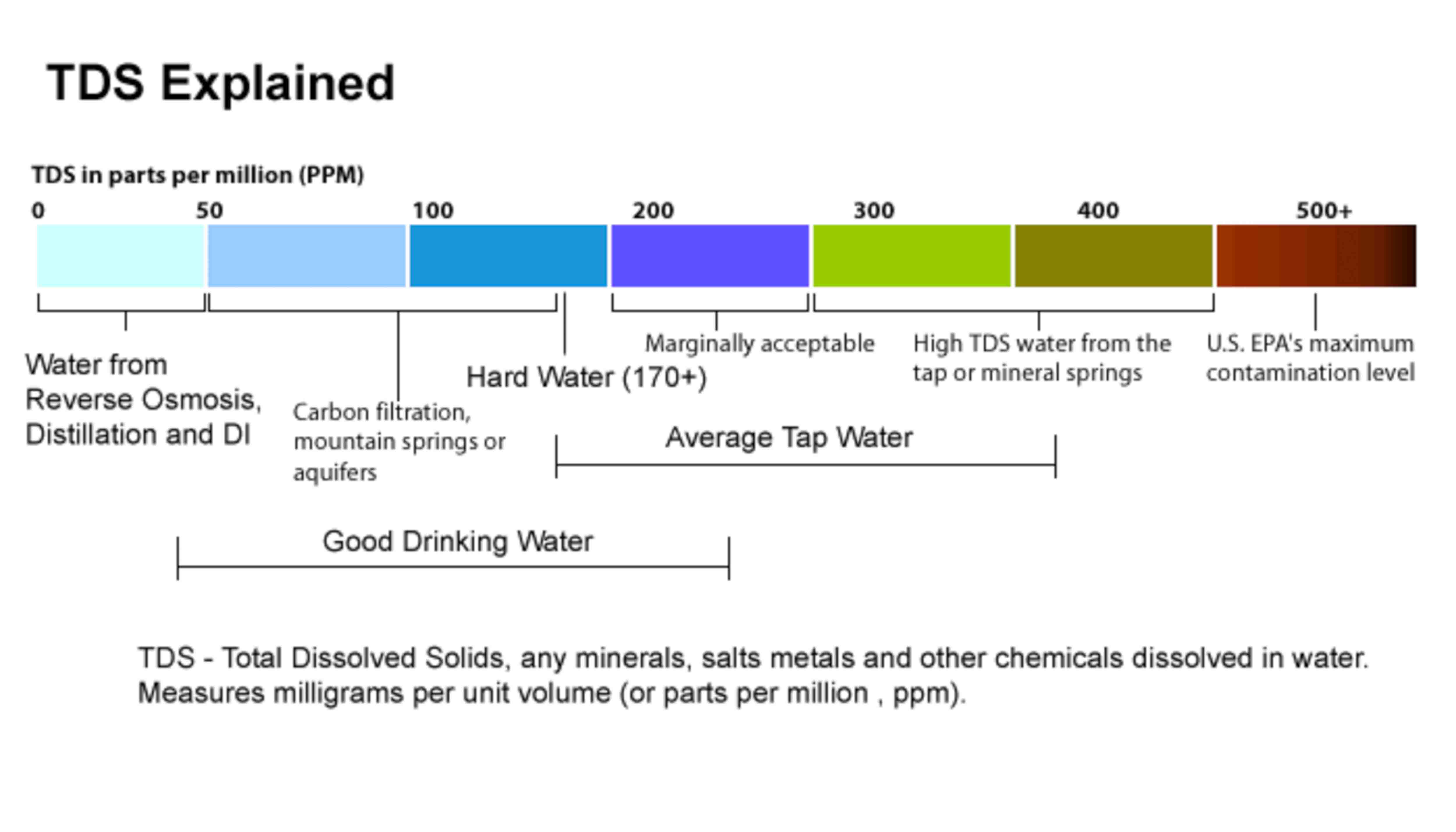
TDS Explained
Elevated total dissolved solids can result in your water having a bitter or salty taste; result in incrustations, films, or precipitates on fixtures; corrosion of fixtures, and reduced efficiency of water filter and equipment.
Water is a good solvent and picks up impurities easily. Pure water is tasteless, colourless, and odourless and is often called the universal solvent. Dissolved solids refer to any minerals, salts, metals dissolved in water. Total dissolved solids (TDS) comprise inorganic salts (principally calcium, magnesium, potassium, sodium, bicarbonates, chlorides, and sulphates) and some small amounts of organic matter that are dissolved in water.
TDS in drinking-water originate from natural sources, sewage, urban run-off, industrial wastewater, and chemicals used in the water treatment process, and the nature of the piping or hardware used to convey the water, i.e., the plumbing. Elevated TDS has been due to natural environmental features such as mineral springs, carbonate deposits, salt deposits.
The total dissolved solids test provides a qualitative measure of the amount of dissolved solids. The test does not provide us insight into the specific water quality issues, such as hardness, salty taste or the corrosiveness. Therefore, the total dissolved solids test is used as an indicator test to determine the general quality of the water.
Total Dissolved Solids Meter is commonly used for analysing the purity of fresh water, and it is used by professionals to determine if tap water purification systems such as reverse osmosis (RO) or reverse osmosis/deionisation (RO/DI) are working properly.
I can recommend a few TDS meters that are reasonably priced and easy to use.






Latosha Mccandrew
10th December 2020 at 1:59 pm
That is a great tip particularly to those fresh to the blogosphere. Simple but very precise information… Appreciate your sharing this one. A must read post!
thebbqbaker.com
16th December 2020 at 8:43 am
Thank you for your comment, appreciate good feedback